Quantum Secrets of Water: Hydrogen Bonding Reveals the Secrets of H₂O
Alternative text: Visualization of water molecules and hydrogen bonds in quantum research.
Water, the basis of life, holds secrets at the molecular level that continue to fascinate scientists. Despite its apparent simplicity, water's unique properties come from complex quantum interactions, particularly hydrogen bonds. Recent advances by scientists at the École Polytechnique Fédérale de Lausanne (EPFL) provide new insights into these quantum effects, which until now have largely been understood only through theoretical models.
Quantum chemistry of hydrogen bonds of water
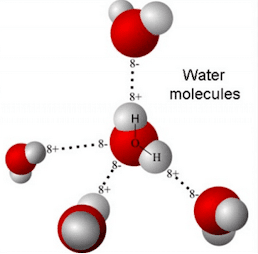
The molecular structure of water, H₂O, is unique in that it can form hydrogen bonds, a phenomenon where hydrogen atoms in one water molecule are attracted to oxygen atoms in neighboring molecules. This bond is responsible for the high surface tension, boiling point, and heat capacity of water. However, quantum effects in these connections are difficult to measure directly.
In a groundbreaking study, scientists led by Sylvie Roque, head of the Fundamental Biophotonics Laboratory at EPFL, developed a technique called correlated vibrational spectroscopy (CVS). This method isolates and measures the interactions of water molecules involved in hydrogen bonds, revealing the distribution of electronic charge and the strength of these bonds in real time.
CVS: a new tool in quantum spectroscopy
Traditional spectroscopy measures the vibrations of all molecules in a sample, making it difficult to distinguish between interacting (bound) and non-interacting molecules. However, CVS allows scientists to capture separate spectra for each type by adjusting the position of the detector and using polarized light. This innovative approach makes it possible to observe molecular interactions that were previously hidden from view.
Using femtosecond laser pulses—short bursts of light that create tiny fluctuations in the charge distribution of water—the researchers were able to detect certain movements in the hydrogen-bonding network. The technique even allows the pH of water to be adjusted by measuring changes in electronic charge due to added ions such as hydroxide and protons, providing unprecedented precision in understanding bond dynamics.
Implications and future research
This breakthrough has far-reaching implications beyond understanding water. As the researchers note, the CVS method can be used to study other complex solutions, such as those containing electrolytes, proteins or DNA. These ideas can contribute to the further development of fields such as biochemistry, pharmacology and materials science.
By unlocking the quantum secrets of water, the EPFL team has discovered new ways to study molecular interactions at the atomic level. This research not only improves our understanding of water, but also demonstrates how advanced spectroscopy can reveal intricate details of how matter behaves.
For more information, see the study in Science by Misha Flora et al. (2024), which contains detailed findings on these quantum interactions and their broader implications.
Sources:
SciTechDaily article the quantum chemistry of water



Comments
Post a Comment